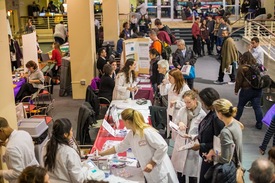
WASHINGTON, March 29, 2016 -- The Center for Information and Study on Clinical Research Participation (CISCRP) is pleased to announce its second AWARE for All event in the D.C. metropolitan area on April 20, 2016. This free program is designed to improve understanding about clinical research participation and promote dialogue by connecting patients, researchers, and members of the general public in the local community.
The event will be hosted at Howard University's Blackburn Center on Wednesday, April 20 from 5 to 8 p.m. in collaboration with Howard University, Howard University Hospital and Georgetown-Howard Universities Center for Clinical and Transitional Science (GHUCCTS). This program will feature free health screenings, informational exhibits, meaningful presentations from prominent D.C. medical professionals and researchers about various health conditions, and local patients involved in clinical research, as well as refreshments and prizes. Funding for this event is provided by PhRMA, EMD Serono, Howard University, Lupus Research Institute, Optimal Research, and other local and national health and advocacy organizations.
"The discovery of new treatments is not possible without volunteers participating in clinical research," said Jocelyn Ulrich, Assistant Vice President, Science and Regulatory Advocacy, PhRMA. "For this reason, it is critical to both educate the public about clinical research and celebrate those who have participated. AWARE for All is an opportunity for this type of community education and engagement, and we're proud to be sponsoring the program for a second year."
|
|||||
CISCRP has held more than 50 of these AWARE for All Clinical Research Education Events in cities around the world, bringing communities together in each destination for important conversations about clinical research and the important role it plays in each of our lives.
"There is a need for general education about the clinical research process including the risks, benefits, and the rights of participants. Ultimately, CISCRP hopes to empower patients and the public to make informed decisions about clinical research." explained Ken Getz, Founder and Chairman of the Board at CISCRP.
AWARE for All is a free event is open to the public; however, advanced registration is strongly encouraged through www.awareforall.org or by calling 1-877-MED-HERO (1-877-633-4370).
ABOUT CISCRP:
The Center for Information and Study (CISCRP) is a 501(c)(3) non-profit organization dedicated to engaging the public and patients as partners in the clinical research process. CISCRP provides free education and outreach to the general public and patient communities. Visit www.CISCRP.org for more information or to support CISCRP's educational initiatives.
Editor's Note
Community partners include: the Arthritis Foundation, Association of Clinical Research Professionals, Bristol-Myers Squibb, Cancer Support Community, Emerson Clinical Research, Familia Con Diabetes, FDA Office of Minority Health, Fuerza Contra Alzheimer's, Georgetown University School of Nursing, The George Washington University School of Medicine and Health Sciences, HealthiVibe, Howard University Clinical Research Unit (CRU), Hudson Global, the Leukemia & Lymphoma Society, Lupus Research Institute, Men's Health Network, The National Coalition for Women with Heart Disease (WomenHeart), Parkinson's Disease Foundation, PhRMA, Society for Women's Health Research, US Against Alzheimer's, and Whitman Walker- AIDS Clinical Trials Group.
A photo accompanying this release is available at: http://www.globenewswire.com/newsroom/prs/?pkgid=39613
CONTACT: Danielle Lavieri, CISCRP
Phone: 617-725-2750
[email protected]



 Nvidia, ByteDance, and the U.S.-China AI Chip Standoff Over H200 Exports
Nvidia, ByteDance, and the U.S.-China AI Chip Standoff Over H200 Exports  Instagram Outage Disrupts Thousands of U.S. Users
Instagram Outage Disrupts Thousands of U.S. Users  Alphabet’s Massive AI Spending Surge Signals Confidence in Google’s Growth Engine
Alphabet’s Massive AI Spending Surge Signals Confidence in Google’s Growth Engine  SpaceX Pushes for Early Stock Index Inclusion Ahead of Potential Record-Breaking IPO
SpaceX Pushes for Early Stock Index Inclusion Ahead of Potential Record-Breaking IPO  Toyota’s Surprise CEO Change Signals Strategic Shift Amid Global Auto Turmoil
Toyota’s Surprise CEO Change Signals Strategic Shift Amid Global Auto Turmoil  Missouri Judge Dismisses Lawsuit Challenging Starbucks’ Diversity and Inclusion Policies
Missouri Judge Dismisses Lawsuit Challenging Starbucks’ Diversity and Inclusion Policies  TSMC Eyes 3nm Chip Production in Japan with $17 Billion Kumamoto Investment
TSMC Eyes 3nm Chip Production in Japan with $17 Billion Kumamoto Investment  Anthropic Eyes $350 Billion Valuation as AI Funding and Share Sale Accelerate
Anthropic Eyes $350 Billion Valuation as AI Funding and Share Sale Accelerate  Prudential Financial Reports Higher Q4 Profit on Strong Underwriting and Investment Gains
Prudential Financial Reports Higher Q4 Profit on Strong Underwriting and Investment Gains  Amazon Stock Rebounds After Earnings as $200B Capex Plan Sparks AI Spending Debate
Amazon Stock Rebounds After Earnings as $200B Capex Plan Sparks AI Spending Debate  SpaceX Prioritizes Moon Mission Before Mars as Starship Development Accelerates
SpaceX Prioritizes Moon Mission Before Mars as Starship Development Accelerates  CK Hutchison Launches Arbitration After Panama Court Revokes Canal Port Licences
CK Hutchison Launches Arbitration After Panama Court Revokes Canal Port Licences  Nasdaq Proposes Fast-Track Rule to Accelerate Index Inclusion for Major New Listings
Nasdaq Proposes Fast-Track Rule to Accelerate Index Inclusion for Major New Listings  Nvidia CEO Jensen Huang Says AI Investment Boom Is Just Beginning as NVDA Shares Surge
Nvidia CEO Jensen Huang Says AI Investment Boom Is Just Beginning as NVDA Shares Surge  Australian Scandium Project Backed by Richard Friedland Poised to Support U.S. Critical Minerals Stockpile
Australian Scandium Project Backed by Richard Friedland Poised to Support U.S. Critical Minerals Stockpile  OpenAI Expands Enterprise AI Strategy With Major Hiring Push Ahead of New Business Offering
OpenAI Expands Enterprise AI Strategy With Major Hiring Push Ahead of New Business Offering  AMD Shares Slide Despite Earnings Beat as Cautious Revenue Outlook Weighs on Stock
AMD Shares Slide Despite Earnings Beat as Cautious Revenue Outlook Weighs on Stock