Lung cancer is responsible for more deaths than breast, colon and prostate cancer combined.
With advancements in lung cancer screening, it is expected that more patients will be diagnosed at earlier stages, enabling them to undergo surgery, the primary treatment modality for early-stage patients.
However, a significant proportion of patients will have a recurrence of their cancer after resection (surgery to remove the tumour). Unfortunately, current clinical guidelines cannot help predict which patients are at risk. Better knowledge of who is at risk has significant implications for systemic therapy selection such as chemotherapy for early-stage lung cancer patients after surgery.
To find solutions to this problem, our research group at McGill University launched a project in collaboration with Université Laval. Preliminary results were published in Nature. In our work we discovered that the use of a new imaging technology, along with artificial intelligence, could improve outcomes for cancer patients.
Too much or too little intervention
This clinical dilemma has important implications for the choice of treatment, such as chemotherapy. For example, lung cancer patients who are cured by surgery could be spared the toxicity of chemotherapy. Patients at risk of their cancer recurring could benefit from additional therapeutic interventions.
The challenge of predicting recurrence for patients with early-stage lung cancer has important implications for the 31,000 Canadians who are diagnosed with this terrible disease every year.
Mass cytometry imaging
To address this clinical problem, we used imaging mass cytometry (IMC), a new technology that allows for a comprehensive characterization of the tumour microenvironment.
The tumour microenvironment is a complex ecosystem composed of interactions between tumour cells, immune cells, and various structural cells. IMC can be used to visualize up to 50 markers at the cell surface, significantly more than was previously possible.
This technology makes it possible to identify different types of cells and determine their spatial organization, i.e. how they interact. IMC produces images that can be analyzed to determine the frequency of cell subpopulations, their activation states, the other cell types with which they interact and their organization in cellular communities.
The results of our study, published in Nature, reveal that various cell types can interact in cellular communities, and that communities composed of B cells were strongly associated with prolonged survival in lung cancer patients. Our study highlights that beyond cellular frequency, cellular interactions and spatial organization also correlate strongly with important clinical outcomes such as survival.
Using artificial intelligence to make better predictions
Based on our initial results, we hypothesized that important spatial features embedded within IMC images, such as cellular interactions, could be important in predicting clinical outcomes.
Our dataset of 416 patients and over 1.6 million cells provided sufficient power to make predictions using artificial intelligence. We sought to predict which patients with early-stage lung cancer would have a recurrence of their cancer after surgery.
Using 1 mm2 tumour samples, material readily available from surgical resections or biopsies, we used artificial intelligence algorithms together with IMC images to make our predictions. Our algorithm was able to predict with 95 per cent accuracy which patients would experience a cancer recurrence by using the spatial information contained within the images.
Six markers can make all the difference
One of the challenges in applying our results in a clinical setting is that IMC is not readily available. Clinical pathologists typically use less complex technologies such as immunofluorescence, which are often limited to three or fewer markers.
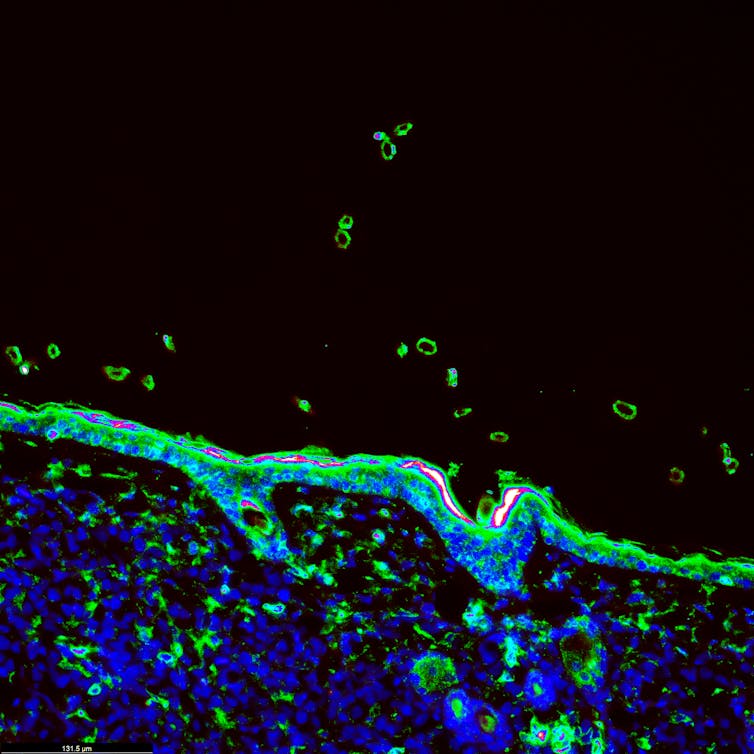
Immunofluorescence image of a tumour treated with immunotherapy. This technology is often limited to the use of three or fewer markers at a time. (Shutterstock)
To address this challenge, we sought to identify the minimum number of markers needed to make meaningful predictions about recurrence in lung cancer patients after surgery. By using six markers, we obtained an accuracy rate of 93 per cent, a result that is close to the 95 per cent accuracy rate obtained by using 35 markers.
These results suggest that by harnessing the power of artificial intelligence with existing technologies available in hospitals, we may be able to improve the post-surgical clinical management of patients with early-stage lung cancer. Our ultimate goal is to increase cure rates for those at high risk of cancer recurrence, while minimizing toxicity for those who can be cured by surgery.



 Sanofi Gains China Approval for Myqorzo and Redemplo, Strengthening Rare Disease Portfolio
Sanofi Gains China Approval for Myqorzo and Redemplo, Strengthening Rare Disease Portfolio  FDA Fast-Tracks Approval of Altria’s on! PLUS Nicotine Pouches Under New Pilot Program
FDA Fast-Tracks Approval of Altria’s on! PLUS Nicotine Pouches Under New Pilot Program  Novo Nordisk Stock Surges After FDA Approves Wegovy Pill for Weight Loss
Novo Nordisk Stock Surges After FDA Approves Wegovy Pill for Weight Loss  AstraZeneca’s LATIFY Phase III Trial of Ceralasertib Misses Primary Endpoint in Lung Cancer Study
AstraZeneca’s LATIFY Phase III Trial of Ceralasertib Misses Primary Endpoint in Lung Cancer Study  Federal Appeals Court Blocks Trump-Era Hospital Drug Rebate Plan
Federal Appeals Court Blocks Trump-Era Hospital Drug Rebate Plan  U.S. Vaccine Policy Shifts Under RFK Jr. Create Uncertainty for Pharma and Investors
U.S. Vaccine Policy Shifts Under RFK Jr. Create Uncertainty for Pharma and Investors  TrumpRx.gov Highlights GLP-1 Drug Discounts but Offers Limited Savings for Most Americans
TrumpRx.gov Highlights GLP-1 Drug Discounts but Offers Limited Savings for Most Americans  Viking Therapeutics Sees Growing Strategic Interest in $150 Billion Weight-Loss Drug Market
Viking Therapeutics Sees Growing Strategic Interest in $150 Billion Weight-Loss Drug Market  Novo Nordisk and Eli Lilly Cut Obesity Drug Prices in China as Competition Intensifies
Novo Nordisk and Eli Lilly Cut Obesity Drug Prices in China as Competition Intensifies  Merck Raises Growth Outlook, Targets $70 Billion Revenue From New Drugs by Mid-2030s
Merck Raises Growth Outlook, Targets $70 Billion Revenue From New Drugs by Mid-2030s  Sanofi to Acquire Dynavax in $2.2 Billion Deal to Strengthen Vaccine Portfolio
Sanofi to Acquire Dynavax in $2.2 Billion Deal to Strengthen Vaccine Portfolio