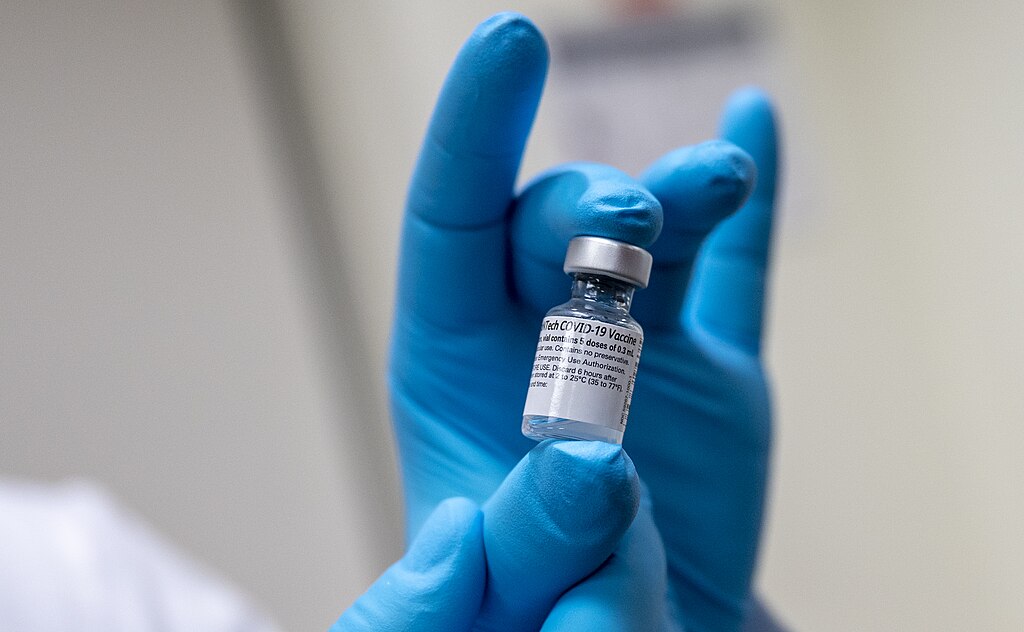
In a groundbreaking decision, a federal judge has mandated the U.S. Food and Drug Administration (FDA) to release an additional one million pages of documents related to Pfizer’s COVID-19 vaccine trials. The data, initially set to remain sealed for 75 years, has become a focal point in debates surrounding transparency and public trust in vaccine safety and efficacy.
The ruling, issued earlier this week, follows a lawsuit filed by the nonprofit group Public Health and Medical Professionals for Transparency. The organization argued that the FDA’s decision to delay the release of critical trial data for decades was both unreasonable and detrimental to public trust. The court’s decision marks another step toward uncovering the details behind one of the most widely administered vaccines in history.
Transparency Push Sparks Controversy
Critics of the FDA’s initial decision to withhold the documents have pointed to the importance of full disclosure, particularly during a global pandemic that has claimed millions of lives. Public interest in the data grew after the agency attempted to release only 500 pages per month, a timeline that would have spanned until 2097.
“The people have a right to know what went into the development and approval of these vaccines,” said Aaron Siri, attorney for the plaintiffs. He emphasized that the accelerated approval process for COVID-19 vaccines heightened the need for oversight and transparency.
However, public health officials have cautioned against misinterpreting the data, warning that incomplete or decontextualized findings could be weaponized by vaccine skeptics. The FDA maintains that its approval process adhered to the highest standards of scientific rigor and integrity.
Pfizer, one of the leading manufacturers of COVID-19 vaccines, has yet to comment on the court’s ruling. The pharmaceutical giant previously stated that its trial data met all regulatory requirements and that its vaccines have been proven safe and effective.
Public Reactions Divide Opinion
The court’s decision has ignited fierce debate online, with social media users expressing a wide range of opinions:
- @TruthSeeker92: “Why hide the data for 75 years if there’s nothing to conceal? Transparency is non-negotiable!”
- @VaxAdvocate: “This ruling will only fuel misinformation. People don’t understand raw data—it’s a dangerous move.”
- @HealthForAll: “Finally, accountability! The FDA owes the public answers for rushing this vaccine.”
- @ScienceFirst: “Releasing the data is fine, but watch how anti-vaxxers twist it into conspiracy theories.”
- @DataDiver: “Can’t wait to analyze the docs. We deserve to see what informed their decisions.”
- @JustAnotherCitizen: “This could lead to unnecessary panic. We need experts to interpret the data before jumping to conclusions.”



 U.S. Vaccine Policy Shifts Under RFK Jr. Create Uncertainty for Pharma and Investors
U.S. Vaccine Policy Shifts Under RFK Jr. Create Uncertainty for Pharma and Investors  California Seeks Court Order to Halt Amazon’s Alleged Price Inflation Practices
California Seeks Court Order to Halt Amazon’s Alleged Price Inflation Practices  FedEx Sues U.S. Government for Refund of Trump-Era Emergency Tariffs After Supreme Court Ruling
FedEx Sues U.S. Government for Refund of Trump-Era Emergency Tariffs After Supreme Court Ruling  CDC Acting Director Urges Measles Vaccination as U.S. Cases Surge in 2026
CDC Acting Director Urges Measles Vaccination as U.S. Cases Surge in 2026  Panama Investigates CK Hutchison’s Port Unit After Court Voids Canal Contracts
Panama Investigates CK Hutchison’s Port Unit After Court Voids Canal Contracts  Mark Zuckerberg Testifies in Youth Social Media Addiction Trial Over Instagram Policies
Mark Zuckerberg Testifies in Youth Social Media Addiction Trial Over Instagram Policies  TrumpRx.gov Highlights GLP-1 Drug Discounts but Offers Limited Savings for Most Americans
TrumpRx.gov Highlights GLP-1 Drug Discounts but Offers Limited Savings for Most Americans  Weight-Loss Drug Ads Take Over the Super Bowl as Pharma Embraces Direct-to-Consumer Marketing
Weight-Loss Drug Ads Take Over the Super Bowl as Pharma Embraces Direct-to-Consumer Marketing  Novo Nordisk Warns of Profit Decline as Wegovy Faces U.S. Price Pressure and Rising Competition
Novo Nordisk Warns of Profit Decline as Wegovy Faces U.S. Price Pressure and Rising Competition  Federal Judge Orders Refund of Trump’s Emergency Tariffs, Potentially Returning Up to $182 Billion
Federal Judge Orders Refund of Trump’s Emergency Tariffs, Potentially Returning Up to $182 Billion  Meta Encryption Plan Sparks Child Safety Concerns Amid New Mexico Lawsuit
Meta Encryption Plan Sparks Child Safety Concerns Amid New Mexico Lawsuit  FDA Fast-Track Drug Reviews Delayed Over Safety and Efficacy Concerns
FDA Fast-Track Drug Reviews Delayed Over Safety and Efficacy Concerns  Peter Mandelson Arrested in London Amid Jeffrey Epstein Ties Investigation
Peter Mandelson Arrested in London Amid Jeffrey Epstein Ties Investigation  ICE Arrests Colombian Journalist in Tennessee, Trump Administration Says She Will Receive Due Process
ICE Arrests Colombian Journalist in Tennessee, Trump Administration Says She Will Receive Due Process  California Court Rejects xAI Bid to Block AI Data Transparency Law
California Court Rejects xAI Bid to Block AI Data Transparency Law