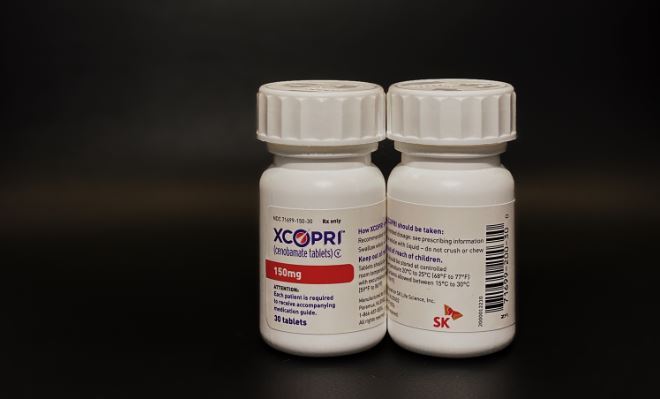
SK Biopharmaceutical is eyeing a share of the 4 trillion won US antiepileptic drug market with its cenobamate tablet Xcopri, which treats partial-onset seizures in adults.
The drug, approved by the US Food and Drug Administration (FDA) on November 21 last year, provides an extra needed treatment option for people with seizures.
Xcopri is the first Korean novel drug, researched and developed entirely and independently by a Korean pharma firm, notes SK Biopharmaceutical CEO Cho.
It is a product of Cho's 15-year research and development drive.
According to the US FDA, the safety and efficacy of XCOPRI were established in two studies that enrolled 655 adults, which were randomized, double-blind, and placebo-controlled.
The FDA granted Xcopri's approval to SK Life Science Inc., the US arm of SK Biopharmaceutical.
Thus, SK Life Science will undertake the US distribution of Xcopri.



 Toyota’s Surprise CEO Change Signals Strategic Shift Amid Global Auto Turmoil
Toyota’s Surprise CEO Change Signals Strategic Shift Amid Global Auto Turmoil  CK Hutchison Launches Arbitration After Panama Court Revokes Canal Port Licences
CK Hutchison Launches Arbitration After Panama Court Revokes Canal Port Licences  Rio Tinto Shares Hit Record High After Ending Glencore Merger Talks
Rio Tinto Shares Hit Record High After Ending Glencore Merger Talks  Once Upon a Farm Raises Nearly $198 Million in IPO, Valued at Over $724 Million
Once Upon a Farm Raises Nearly $198 Million in IPO, Valued at Over $724 Million  Hims & Hers Halts Compounded Semaglutide Pill After FDA Warning
Hims & Hers Halts Compounded Semaglutide Pill After FDA Warning  SoftBank Shares Slide After Arm Earnings Miss Fuels Tech Stock Sell-Off
SoftBank Shares Slide After Arm Earnings Miss Fuels Tech Stock Sell-Off  Ford and Geely Explore Strategic Manufacturing Partnership in Europe
Ford and Geely Explore Strategic Manufacturing Partnership in Europe  Tencent Shares Slide After WeChat Restricts YuanBao AI Promotional Links
Tencent Shares Slide After WeChat Restricts YuanBao AI Promotional Links  Uber Ordered to Pay $8.5 Million in Bellwether Sexual Assault Lawsuit
Uber Ordered to Pay $8.5 Million in Bellwether Sexual Assault Lawsuit  Global PC Makers Eye Chinese Memory Chip Suppliers Amid Ongoing Supply Crunch
Global PC Makers Eye Chinese Memory Chip Suppliers Amid Ongoing Supply Crunch  Baidu Approves $5 Billion Share Buyback and Plans First-Ever Dividend in 2026
Baidu Approves $5 Billion Share Buyback and Plans First-Ever Dividend in 2026  TrumpRx Website Launches to Offer Discounted Prescription Drugs for Cash-Paying Americans
TrumpRx Website Launches to Offer Discounted Prescription Drugs for Cash-Paying Americans  Nvidia CEO Jensen Huang Says AI Investment Boom Is Just Beginning as NVDA Shares Surge
Nvidia CEO Jensen Huang Says AI Investment Boom Is Just Beginning as NVDA Shares Surge  American Airlines CEO to Meet Pilots Union Amid Storm Response and Financial Concerns
American Airlines CEO to Meet Pilots Union Amid Storm Response and Financial Concerns  SpaceX Prioritizes Moon Mission Before Mars as Starship Development Accelerates
SpaceX Prioritizes Moon Mission Before Mars as Starship Development Accelerates  Trump Backs Nexstar–Tegna Merger Amid Shifting U.S. Media Landscape
Trump Backs Nexstar–Tegna Merger Amid Shifting U.S. Media Landscape  Weight-Loss Drug Ads Take Over the Super Bowl as Pharma Embraces Direct-to-Consumer Marketing
Weight-Loss Drug Ads Take Over the Super Bowl as Pharma Embraces Direct-to-Consumer Marketing