In the last decade, it has become increasingly clear that a gut full of friendly microbes (the microbiome) is vital for our good health. It has also become clear that a healthy microbiome is one with a diverse population of microbes (bacteria, viruses, fungi). What we haven’t known is why a diverse array of friendly bugs is important for keeping the harmful microbes (pathogens) at bay. But now we think we have found the answer.
Our latest study, published in Science, shows that the main reason a diverse microbiome is helpful for resisting pathogens is that the friendly microbes collectively consume the nutrients needed for a pathogen to grow in the gut.
In other words, a diverse microbiome blocks pathogen growth by using its nutrients – a phenomenon we call “nutrient blocking”. Understanding how nutrient blocking works is powerful because it allows us to predict which gut communities will be protective against a given pathogen.
Our research team started by conducting a large screen of 100 common strains of human gut bacteria. We tested the individual ability of these strains to restrict the growth of two bacterial pathogens, Klebsiella pneumoniae and Salmonella enterica serovar Typhimurium. These two species are a problem because many strains are becoming resistant to antibiotics.
Alone, the gut strains could not prevent pathogen growth. However, this was different when several of these species were pooled into larger communities. Higher diversity communities tended to offer more protection against invading pathogens, both in test-tube experiments and when tested in mice.
However, not all higher diversity communities tested restricted pathogen growth. Crucially, certain species must be present for a community to be protective.
We then went on to investigate the reason why. We compared the nutrients that could be consumed by each of the individual species with the nutrients consumed by the pathogens. We realised that certain gut species had a higher nutrient use overlap with the pathogens than other species.
Still, individually, the nutrient use overlap of even these key species with the pathogens was not high enough to block pathogen growth. But when we considered which nutrients the community as a whole can use, we found that the communities with the highest degree of overlap with the pathogens provided the most protection.
Suggesting treatments
This finding is important as it provides a route for the design of beneficial, probiotic communities that aim to boost the microbiome’s ability to resist colonisation by disease-causing pathogens.
We tested this idea by taking a set of 50 human gut species and predicting which combinations of species would be protective against a new pathogen: a drug-resistant Escherichia coli.
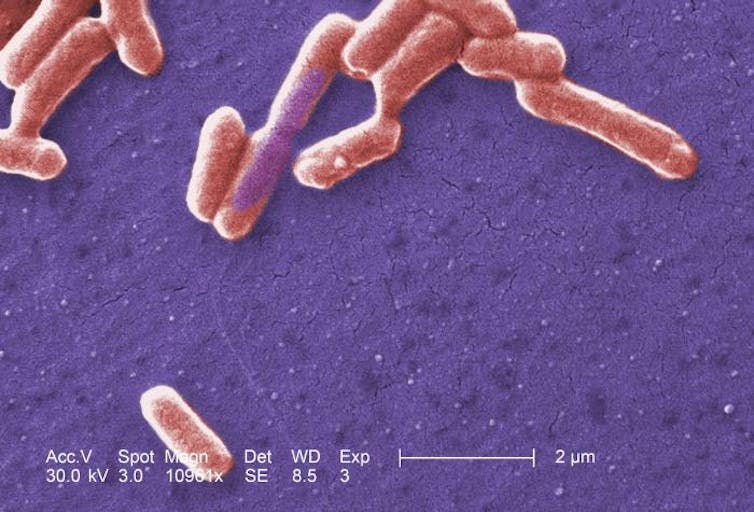
Some strains of E coli bacteria (seen here) cause us harm. CDC/Janice Haney Carr
We selected the communities that were predicted to be poor at blocking the growth of this pathogenic E coli isolate, and communities that were predicted to be good, and tested them against the new pathogen. In all cases, the nutrient blocking principle was able to successfully predict the ability of the community to block the growth of the pathogen.
Antibiotic treatment failure is becoming more common due to the spread of resistant pathogens. Alternative solutions that harness the body’s natural ability to resist disease, such as by tinkering with the microbiome’s composition, are becoming more attractive.
The gut microbiome is integral to our health, but it is very complex and therefore hard to understand. Our study provides a template for how one might rationally design probiotic communities to engineer microbiomes for better health.



 Sanofi Reports Positive Late-Stage Results for Amlitelimab in Eczema Treatment
Sanofi Reports Positive Late-Stage Results for Amlitelimab in Eczema Treatment  FDA Fast-Track Drug Reviews Delayed Over Safety and Efficacy Concerns
FDA Fast-Track Drug Reviews Delayed Over Safety and Efficacy Concerns  Novo Nordisk and Eli Lilly Cut Obesity Drug Prices in China as Competition Intensifies
Novo Nordisk and Eli Lilly Cut Obesity Drug Prices in China as Competition Intensifies  Eli Lilly and Novo Nordisk Battle for India’s Fast-Growing Obesity Drug Market
Eli Lilly and Novo Nordisk Battle for India’s Fast-Growing Obesity Drug Market  RFK Jr. Overhauls Federal Autism Panel, Sparking Medical Community Backlash
RFK Jr. Overhauls Federal Autism Panel, Sparking Medical Community Backlash  AstraZeneca’s LATIFY Phase III Trial of Ceralasertib Misses Primary Endpoint in Lung Cancer Study
AstraZeneca’s LATIFY Phase III Trial of Ceralasertib Misses Primary Endpoint in Lung Cancer Study  Merck Raises Growth Outlook, Targets $70 Billion Revenue From New Drugs by Mid-2030s
Merck Raises Growth Outlook, Targets $70 Billion Revenue From New Drugs by Mid-2030s  Novo Nordisk Launches Once-Daily Wegovy Pill in U.S. at Competitive Pricing
Novo Nordisk Launches Once-Daily Wegovy Pill in U.S. at Competitive Pricing  Sanofi to Acquire Dynavax in $2.2 Billion Deal to Strengthen Vaccine Portfolio
Sanofi to Acquire Dynavax in $2.2 Billion Deal to Strengthen Vaccine Portfolio  Weight-Loss Drug Ads Take Over the Super Bowl as Pharma Embraces Direct-to-Consumer Marketing
Weight-Loss Drug Ads Take Over the Super Bowl as Pharma Embraces Direct-to-Consumer Marketing