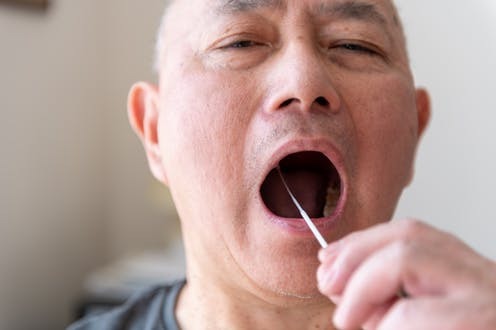
Flu season has begun uncharacteristically early this year, and so far we’ve seen around 187,000 laboratory-confirmed cases, 1,323 hospitalisations, and 113 deaths.
The risk of infection from either COVID or influenza this winter will be very high. The risk of being infected with both at the same time will also be significant, and will likely put a huge strain on our already overburdened health system.
A large number of people who get the flu do not get tested, unless the symptoms are severe. Early detection of flu can improve treatment to prevent significant illness, particularly in the young, elderly and immunocompromised.
A simple RAT test, the same as the ones we’ve become accustomed to using for COVID, could potentially help in detecting the virus early, and stopping the spread of flu.
How do flu RATs work?
RATs are short for rapid antigen tests and they can quickly test whether a person has Influenza A or B – two of the most common strains of flu virus.
The test is designed to pick up the presence of specific antigens of the flu virus, which when detected produce a coloured band to indicate a positive test result, similar to the line you’ve seen on your COVID RATs.
At the moment, RAT tests for flu are not widely available, are expensive compared to the COVID tests, and most suffer problems with sensitivity and/or specificity (their ability to detect positive cases) that need further work and testing. However, this could change if there was more demand for flu RATs.
Antivirals reduce the risk of hospitalisation in flu patients. Shutterstock
Could flu RATs make a different to rates of flu and illness from flu?
Influenza and COVID cause similar symptoms, however, the drugs we have to manage these diseases require a precise identification. This is critical, as it governs what type of antiviral drug is given.
Current flu RATs are most sensitive up to four days after symptom onset. A positive detection during this period can facilitate a quicker treatment strategy with antivirals, as these drugs have a narrow window of therapeutic opportunity.
If a patient tests positive for flu, they can then receive Tamiflu, the antiviral recommended for flu cases, which reduces the risk of hospitalisation.
However this must be taken within two days of infection (when symptoms emerge) for it to be effective. Everyone can get Tamiflu but they require a prescription, which makes it difficult to get the drug within the two-day window needed to be effective.
Tamiflu would not work against COVID, which requires a different antiviral for elderly and otherwise unwell patients, such as Paxlovid.
A flu RAT would benefit people at risk of severe illness such as babies, young children, the elderly and the immunocompromised. This would increase the chance of early detection to enable treatment, and it could also give us more accurate figures of the number of people with flu each season.
Flu RATs could also help in the management of outbreaks in high-risk communities such as aged care, nursing homes, schools and day care. A quick detection of flu could assist in measures to reduce the chances of transmission by antiviral treatment and isolation, as we’ve seen with COVID.
Are flu RATs available in Australia?
There are currently no flu RATs approved by the Therapeutic Goods Administration (TGA) in Australia, for public use at home. The TGA has approved eight tests that are used only in a clinical setting, and these are designed to detect both flu and COVID.
We need to lobby the government to action TGA approval of home flu RATs, as public demand will help drive this process. The TGA will require time and money to test and develop the RATs to a high standard.
Therefore we can’t expect Australian-based RATs for this flu season, but given we are now all so comfortable with at-home antigen testing, testing for flu is the logical next step.



 Sanofi to Acquire Dynavax in $2.2 Billion Deal to Strengthen Vaccine Portfolio
Sanofi to Acquire Dynavax in $2.2 Billion Deal to Strengthen Vaccine Portfolio  FDA Approves Mitapivat for Anemia in Thalassemia Patients
FDA Approves Mitapivat for Anemia in Thalassemia Patients  Viking Therapeutics Sees Growing Strategic Interest in $150 Billion Weight-Loss Drug Market
Viking Therapeutics Sees Growing Strategic Interest in $150 Billion Weight-Loss Drug Market  AstraZeneca’s LATIFY Phase III Trial of Ceralasertib Misses Primary Endpoint in Lung Cancer Study
AstraZeneca’s LATIFY Phase III Trial of Ceralasertib Misses Primary Endpoint in Lung Cancer Study  Novo Nordisk and Eli Lilly Cut Obesity Drug Prices in China as Competition Intensifies
Novo Nordisk and Eli Lilly Cut Obesity Drug Prices in China as Competition Intensifies  Royalty Pharma Stock Rises After Acquiring Full Evrysdi Royalty Rights from PTC Therapeutics
Royalty Pharma Stock Rises After Acquiring Full Evrysdi Royalty Rights from PTC Therapeutics  Sanofi Gains China Approval for Myqorzo and Redemplo, Strengthening Rare Disease Portfolio
Sanofi Gains China Approval for Myqorzo and Redemplo, Strengthening Rare Disease Portfolio  Novo Nordisk Launches Once-Daily Wegovy Pill in U.S. at Competitive Pricing
Novo Nordisk Launches Once-Daily Wegovy Pill in U.S. at Competitive Pricing  Merck Raises Growth Outlook, Targets $70 Billion Revenue From New Drugs by Mid-2030s
Merck Raises Growth Outlook, Targets $70 Billion Revenue From New Drugs by Mid-2030s  RFK Jr. Overhauls Federal Autism Panel, Sparking Medical Community Backlash
RFK Jr. Overhauls Federal Autism Panel, Sparking Medical Community Backlash  Federal Appeals Court Blocks Trump-Era Hospital Drug Rebate Plan
Federal Appeals Court Blocks Trump-Era Hospital Drug Rebate Plan