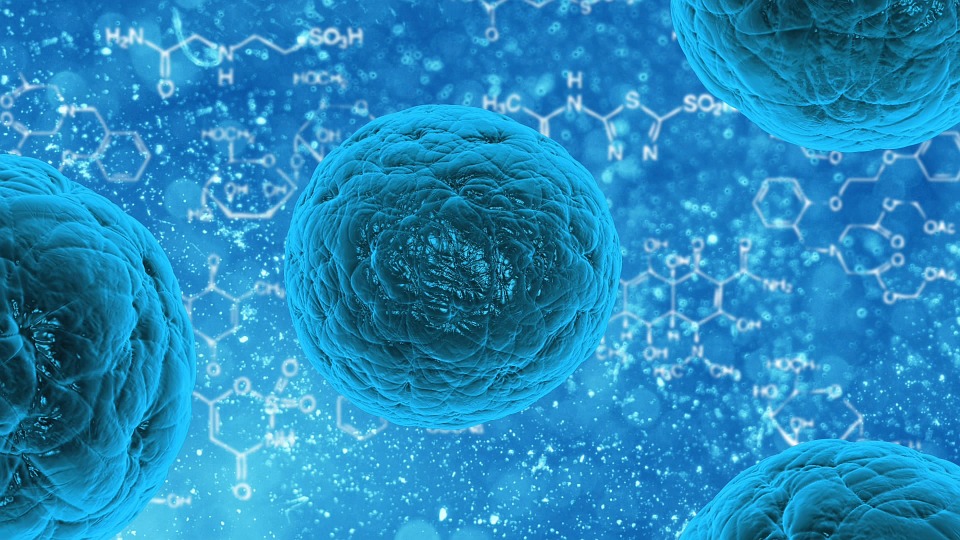
When discussing stem cell research, most people would likely bring up scientists harvesting stem cells from living tissue. While this is certainly true, there is now a new method that would allow researchers to manufacture stem cells so that they have much more of it to work with. The U.S. Food and Drug Administration (FDA) recently approved the process employed by the Mayo Clinic, which actually automates the process.
In a press release, the Mayo Clinic describes both the process and implications of the FDA approval to stem cell research. Apparently, this allows the researchers to take the bone marrow of a donor and then produce a much higher quantity of stem cells that will then be used to advance research in regenerative medicine.
According to the medical director of Transfusion Medicine and the Human Cell Therapy Laboratory, Abba Zubair, this process is a huge boon for cases where the patient’s own stem cells are not enough to allow for treatment. These cases can often be a problem, mostly because harvesting stem cells is not exactly that easy.
“This may make treatments possible in cases where the patient’s own cells are not viable as therapy,” Dr. Zubair said. “In addition, because the cells can be produced in days instead of months, it may also make treatments available on short notice when they’re needed for acute care.”
As Futurism notes, stem cell research has already proven promising in a variety of treatment cases, addressing everything from arthritis to paralysis. By cranking up production of stem cells via an automated production method, not only will researchers have significantly more batches to work with, patients won’t have to wait for months to be treated.
On a side note, this process also highlights the huge advantages to automating the manufacturing process in the field of research and medicine. In these areas, robots are just taking over, and that might be for the best.



 Google Cloud and Liberty Global Forge Strategic AI Partnership to Transform European Telecom Services
Google Cloud and Liberty Global Forge Strategic AI Partnership to Transform European Telecom Services  Tencent Shares Slide After WeChat Restricts YuanBao AI Promotional Links
Tencent Shares Slide After WeChat Restricts YuanBao AI Promotional Links  SpaceX Seeks FCC Approval for Massive Solar-Powered Satellite Network to Support AI Data Centers
SpaceX Seeks FCC Approval for Massive Solar-Powered Satellite Network to Support AI Data Centers  Sony Q3 Profit Jumps on Gaming and Image Sensors, Full-Year Outlook Raised
Sony Q3 Profit Jumps on Gaming and Image Sensors, Full-Year Outlook Raised  Global PC Makers Eye Chinese Memory Chip Suppliers Amid Ongoing Supply Crunch
Global PC Makers Eye Chinese Memory Chip Suppliers Amid Ongoing Supply Crunch  SpaceX Starship Explodes in Texas During Test, Citing Nitrogen Tank Failure
SpaceX Starship Explodes in Texas During Test, Citing Nitrogen Tank Failure  SpaceX Updates Starlink Privacy Policy to Allow AI Training as xAI Merger Talks and IPO Loom
SpaceX Updates Starlink Privacy Policy to Allow AI Training as xAI Merger Talks and IPO Loom  NASA Astronauts Wilmore and Williams Recover After Boeing Starliner Delay
NASA Astronauts Wilmore and Williams Recover After Boeing Starliner Delay  OpenAI Expands Enterprise AI Strategy With Major Hiring Push Ahead of New Business Offering
OpenAI Expands Enterprise AI Strategy With Major Hiring Push Ahead of New Business Offering  Neuralink Plans High-Volume Brain Implant Production and Fully Automated Surgery by 2026
Neuralink Plans High-Volume Brain Implant Production and Fully Automated Surgery by 2026  Jensen Huang Urges Taiwan Suppliers to Boost AI Chip Production Amid Surging Demand
Jensen Huang Urges Taiwan Suppliers to Boost AI Chip Production Amid Surging Demand  Nvidia Confirms Major OpenAI Investment Amid AI Funding Race
Nvidia Confirms Major OpenAI Investment Amid AI Funding Race  Amazon Stock Rebounds After Earnings as $200B Capex Plan Sparks AI Spending Debate
Amazon Stock Rebounds After Earnings as $200B Capex Plan Sparks AI Spending Debate  NASA and SpaceX Target Crew-11 Undocking From ISS Amid Medical Concern
NASA and SpaceX Target Crew-11 Undocking From ISS Amid Medical Concern